
Your product, Our expertise,Your success.
Our team of experts is characterized by its professionalism and its sense of ethics. The plurality of our profiles and personalities makes it possible to support you throughout your projects, for every type of medical device or therapeutic areas.
Thanks to our wide range of skills, our services cover: the submission of reimbursement files, applications for derogatory financing, efficiency files, price negotiations and budget impact analysis. MedConsult ensures the French market access or economic evaluation projects of your technology all along.

Benoit Salaün
Founder and CEO
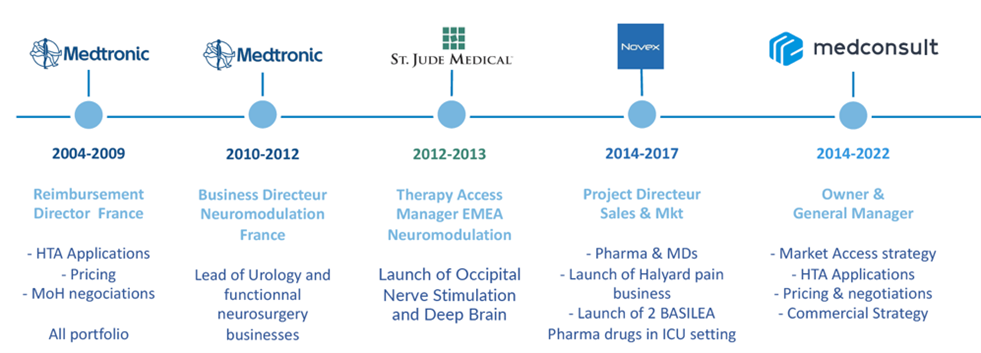
Benoit SALAÜN (PharmD, PhD) is the founder and CEO of Medconsult.
He is an experienced professional with a strong track record in Market Access and Business Operations for medical devices and drugs
As a recognized expert in this field, Benoit is dedicated to satisfying its clients needs.

Nolwenn Clément-Rio
Senior Consultant Market-Access and Medico-Economics
Nolwenn is senior market access and medico-economics consultant at MedConsult. She is pharmD (University Paris Sud) and holds a master’s degree in Market-Access and Medico-Economic Evaluation (University Paris Sud). She also holds a statistics degree (CESAM certification – University Paris VI), specialized in clinical trials and statistics software (R and SAS).
Since joining MedConsult in 2018, Nolwenn has participated in the development of pricing and positioning strategy. She was also involved in the drafting and filing of medico-technical and economic files for registrations on the LPPR. Due to her statistical background, she has been able to conduct analyzes of administrative databases as well as epidemiological monitoring. Plus, she’s leading Delphi Panel projects.

Nathalie Préaubert
Health economics Senior consultant
Nathalie is senior consultant in charge of MedConsult’s “Health economics” department.
She holds a master’s degree in health economics and a another one in public health. Nathalie has worked as health economist at the National institute of medical research (INSERM) then, within the “Economic Evaluation and Public Health” division (SEESP) of the National Health Agency (HAS) for 9 years. In 2009, Nathalie joined the Clinical Research Department of the Bordeaux University Hospital, to manage “the health economic evaluation” division until September 2021, date of her arrival at Medconsult.
The main Nathalie’s mission is to provide medical device and drug manufacturers, an analysis of the economic, budgetary, and organizational issues of their technologies. Nathalie helps them to identify the authorities’ requirements in terms of economic data to be collected for the market access strategy (cost-effectiveness models, economic arguments, budget impact analyses), and to define, set up and follow economic studies implemented. Thanks to her experience in clinical research and in the methodology and statistical analyzes of trials, Nathalie also provides support to the Market Access teams on these aspects.

Valérie Tochon
Senior Consultant Market-Access
Valérie is a senior consultant in Market Access at MedConsult.
She has a degree in Biological Engineering (Polytech Nice Sophia), a master’s degree in Management of Pharmaceutical Industries and MedTechs from the IAE Lyon (University Lyon III) and a certification in Medical Statistics (CESAM – University Paris VI) with a specialization in Epidemiology. Valérie has a solid experience in the reimbursement and market access of health products. Since 2004, she has held various positions in international pharmaceutical and biotechnology companies (Aventis, Novartis, Pfizer, Amgen), always in connection with the evaluation, funding, and reimbursement of health care products. She has developed a strong expertise in market access strategies including regulatory, clinical, medico-economic and commercial aspects. Since 2013, Valérie has been a consultant and has assisted many MedTech companies in the market access process of their product. Initially working as a freelance consultant (Valua), she joined MedConsult in 2022.
Valérie’s mission is to assist medical device companies in understanding the environment and funding channels for healthcare products in France, to conduct situational analyses, to develop market access strategies, to manage the drafting and filing of medico-technical and economic dossiers and to follow up with the health authorities and payers.

Marion Corthier
Market-access consultant
PharmD (University Paris Sud) and holds a master’s degree in Market-Access and Medico-Economic Evaluation (University Paris Sud).
Since joining MedConsult, she has been involved in many various market access missions. Her good knowledge of the French authorities, of the device regulations and of the medical device pathway has enabled her to manage relevant strategic analyses for innovative devices.

Hélène Besseau
Market-access consultant
Hélène joined Medconsult in February 2021 and is a Market Access Consultant. She graduated from the University of Pharmacy in Nantes and also holds a Master’s degree in “Market Access and Medico-Economic Evaluation” from the University of Paris-Saclay.
Her knowledge of the medical device landscape enables her to carry out relevant strategic analyses in order to guide companies towards the best reimbursement pathway for their innovative devices. She is also in charge of writing medico-technical and economic files.

Hela Ayari
Market-access consultant
Hela is a PharmD, who completed her studies with a Master degree “Medico-economic evaluation and market access” at the University of Paris Dauphine.
After 3 years of experience in consulting at Creativ-ceutical for big pharmas, Hela joined Medconsult in June 2021.
Since her arrival, she has been involved in numerous market access projects (strategic recommendations, development of medico-technical and economic dossiers…). Hela is also part of the team “Health Economics Evaluation” of Medconsult.

Sullyvan Plaisir
Market-access consultant
Sullyvan joined MedConsult in April 2022 and is a Market-Access Consultant. In his last year of pharmacy studies at the University of Nancy, he is currently in Master 2 “M2 Medical Devices: Evaluation, Registration, Vigilance” at the University of Paris-Saclay.
His autonomy, his curiosity and his sense of rigor allow him to improve his knowledge within our team in order to acquire rich skills. Since his arrival, he has been involved in numerous missions in market access (analysis and strategic recommendations, or drafting of registration files on the LPPR).

Coline Chea
Market-access consultant
Coline, a Doctor of Pharmacy who completed her studies with a Master’s degree in “Market Access and Health Economics Evaluation” from the University of Paris-Saclay has joined our team in October 2023.
With one and a half years of experience at Boston Scientific, she brings with her a strong expertise that makes her a valuable asset to our team.

Amal Oubouch
Market-access consultant
Amal is a Market Access Consultant at Medconsult. In her final year of pharmacy studies at the University of Paris-Saclay, Amal is preparing to start her Master 2 in “Market Access & Medico-Economic Evaluation”. She is actively involved in a number of market access assignments, including strategic analyses and drafting dossiers for LPPR application.

Yohan Vitali
Assistant Office Manager
Alterning in 2nd of BTS SAM (support for managerial action) Yohan finished his degree at the Etienne Jules Marey high school in order to specialize in the near future in the field of Human Resources.
Since his arrival, he has been involved in many administrative missions such as email tracking, billing tracking, computer park follow-up, filing and archiving of administrative documents, and contract preparation. He is also part of the administrative center of Medconsult.

Schelsea Fifi
Accounting assistant
Schelsea is in her first year of a Master’s degree in Finance at the ESAM of the IGS group. Since her arrival in January 2022, she has been working in the accounting and financial department of MedConsult.
Her availability, her autonomy and her versatility allow her to carry out various missions. Part of her missions include following up the accounting, managing the invoicing, and filing all the supporting documents. She is MedConsult’s main contact for all matters related to invoicing.
